- There are no more items in your cart
- Shipping
- Total 0.00 zł
pH is one of the key parameters that determine the properties of water. Knowledge of its level allows for the assessment of liquid quality, thereby ensuring safety. The pH scale, which indicates the acidity or alkalinity of water, is used in many fields, from chemistry and biology to medicine, agriculture, and industry. In the context of a changing climate and increasing urbanization, monitoring pH is becoming increasingly important to ensure human health and protection.environments.
Last updated: August 26, 2025
In this article, you will learn…
- What does pH mean?
- What does the pH scale look like?
- What are acids and bases?
- What is definition of acids?
- What is the definition of bases?
- What is the significance of pH in the context of water?
- What is the pH of drinking water?
- What is the pH of water in the environment?
- What is pH in water treatment processes?water?
- How to measure pH?
- What is the pH of water – summary?
What does pH mean?
The simplest definition of pH states that it is a measure of the acidity or alkalinity of an aqueous solution. The abbreviation pH comes from the Latin term "potentia hydrogenii", which can be"hydrogen potential." It is the decimal logarithm of the inverse of the concentration of hydrogen ions (H⁺) in a solution.
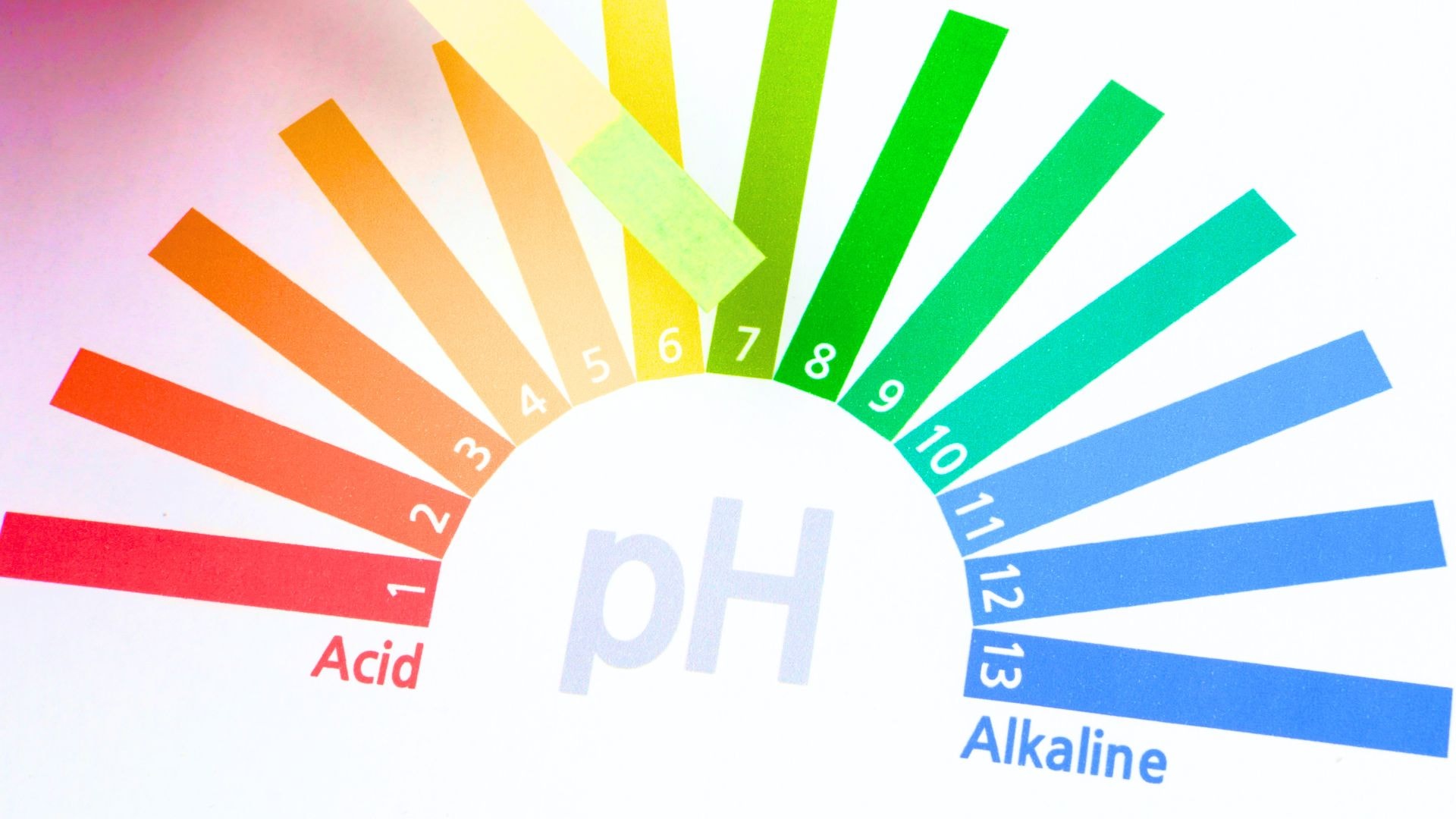
What does the pH scale look like?
The pH scale ranges from 0 to 14, where:
- pH 7 indicates a neutral solution,
- pH below 7 indicates an acidic solution,
- pH above 7 indicates aalkaline solution.
The pH scale is logarithmic, which means that each full unit on this scale represents a tenfold change in the concentration of hydrogen ions. For example, a solution with a pH of 6 is ten times more acidic than a solution with a pH of 7, and a solution with a pH of 5 is one hundred times more acidic than a solution with a pH of 7. Thanks to the logarithmic pH scale, we can accurately measure and compare even very small differences in acidity.solutions.
It is worth remembering: Acids are chemical substances that release hydrogen ions (H⁺) in aqueous solutions.
What are acids and bases?
Definition of acids
Acids are chemical substances that release hydrogen ions (H⁺) in aqueous solutions. The classical definition of an acid has beenproposed by Svante Arrhenius in 1887, and according to it, an acid is a substance that increases the concentration of hydrogen ions in an aqueous solution. Examples of acids include:
- hydrochloric acid (HCl),
- acetic acid (CH₃COOH),
- sulfuric acid (H₂SO₄).
Definition of bases
Bases are substanceschemicals that, in aqueous solutions, accept hydrogen ions or donate hydroxide ions (OH⁻). Arrhenius defined bases as substances that increase the concentration of hydroxide ions in an aqueous solution. Examples of bases include:
- sodium hydroxide (NaOH),
- ammonia (NH₃),
- calcium hydroxide (Ca(OH)₂).

The Importance of pH in the Context of Water
pH of Drinking Water
Drinking water should have a pH in the range of 6.5 to 8.5 to be safe for consumption. Too low pH (acidic water) can cause corrosion of plumbing and the release of heavy metals, such as lead and copper, into the water. Conversely, excessively high pH (alkaline water) can lead to the formation of deposits.mineral content in pipes and the unpleasant taste of water. Additionally, the pH of drinking water can affect its organoleptic properties, such as odor and taste, which is important for consumers.
pH of water in the environment
The pH of water is also significant for aquatic ecosystems. Water with extremely low or high pH can be harmful to aquatic organisms, such as fish, plants, and microorganisms. For example, it canacid rain (precipitation with low pH), which leads to the acidification of lakes and rivers, negatively affecting aquatic life. Changes in pH in surface waters can also cause changes in biodiversity, which is crucial for the stability of ecosystems.
pH in water treatment processes
pH control is crucial in water treatment processes. For example, coagulation and flocculation, which are used toremoving contaminants from water is most effective within a specific pH range. Regulating this parameter is also important during water chlorination. This is due to the fact that disinfection efficiency depends on the water's pH. Additionally, monitoring pH is crucial in the context of public health, as improper pH values can lead to ineffective removal of pathogens.
It's worth remembering: The appropriate pH for drinking water iskey to health and preventing pipe corrosion.
How to measure pH?
There are several methods for measuring pH, which vary in accuracy and application. The most popular devices are:
- Indicator papers – strips soaked in a chemical substance that changes color depending on the pH of the solution. Indicator papers are a simple anda quick way to estimate pH, but their accuracy is limited.
- Chemical indicators – substances that change color depending on the pH of the solution. They are used in laboratories for more precise measurements than indicator papers.
- pH meters – electronic devices that measure pH using an electrode immersed in the solution. These are the most precise toolsfor measuring pH and are widely used in laboratories, industry, and in monitoring the quality of drinking water.

pH of water – summary
pH is a very important parameter that has wide applications in various fields of life, from the quality of drinking water, through agriculture, medicine, to industry. Its monitoring and regulation allow for maintaining water withappropriate parameters, which have a direct impact on human health, the environment, and production processes. In the era of climate change, understanding the role of pH in water becomes crucial for sustainable water resource management.
Frequently Asked Questions (FAQ)
What is pH?
pH is a measure of the acidity or alkalinity of an aqueous solution.
What are the extreme pH values?
The pH scale ranges from 0 to 14, where 0 indicates a strongly acidic solution and 14 a strongly alkaline one.
Why is pH important in drinking water?
The appropriate pH of drinking water is crucial for health and preventing pipe corrosion.
What are the effects of acidic water?
Acidic water can lead to pipe corrosion andthe release of heavy metals.
What devices are used to measure pH?
The most popular are indicator strips, chemical indicators, and pH meters.
What pH is considered neutral?
pH 7 is considered neutral.
Author: MaciejWaliduda — an expert in water quality and treatment technology. He has many years of experience in monitoring and analyzing water parameters.